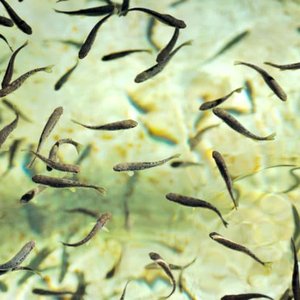
RPS Biologiques received two notification numbers from Health Canada’s Veterinary Drugs Directorate (VDD) approving Puralyze™ in both liquid and powdered forms. The approval allows this novel natural product to be marketed and sold in Canada.
RPS Biologiques develops innovative biotech products with proven market potential. In 2019, the company’s natural product Supratect™ became the first VHP notified by Health Canada for use in aquatic species, an exceptional milestone that paved the way for future technologies to further enrich the aquaculture industry with new, innovative products.
Puralyze™ is a plant-based immersive bath for maintaining the optimal health of fish. It is a safe, effective and sustainable alternative to existing products. Subrata Chowdhury, president of RPS Biologiques, said Puralyze™ is the next step in the company’s commitment to creating first-of-kind biotech products and solutions for aquatic, animal and human health. “Health Canada is widely respected as a regulatory body, and we expect these notifications will help fast track approval of Puralyze™ in international aquaculture markets. We already have overseas customers asking for the product,” said Chowdhury.
The testing to validate Puralyze™ was completed mainly at the Huntsman Marine Science Centre located in St. Andrews, New Brunswick. Established in 1969, Huntsman is a private, not-for-profit research and science-based teaching institution whose work is recognized throughout Canada and internationally. Paul Dick & Associates (PDA), a global consulting company, worked with RPS Biologiques and Health Canada to obtain notification for Puralyze™ via the VHP program. The firm specializes in the development and registration of animal and human health products including veterinary biologics, veterinary drugs, feeds and natural health products.