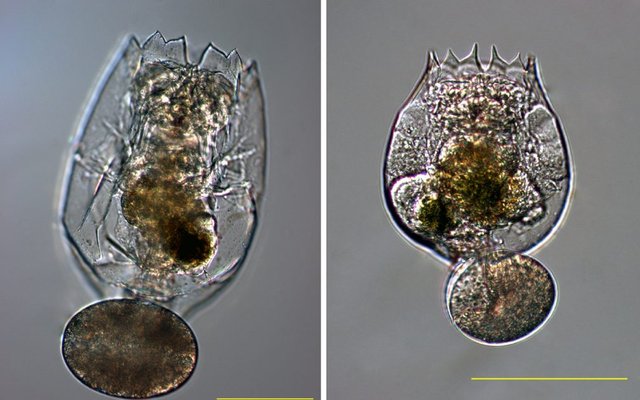
Rotifers have played a critical role in fish hatchery success. The evolution of rotifer culture techniques, from phytoplankton-based systems to high-density continuous cultures, has significantly enhanced larval survival rates in hatcheries and allowed the commercial production of many different species that are farmed today.
At Larvi 2024, Hatchery Feed & Management talked with Atsushi Hagiwara, Professor Emeritus at Nagasaki University and one of the major experts in rotifers, about the advances in rotifer culture and the path to sustainable fish hatcheries.
HFM: You've focused much of your research on rotifers and aquaculture, but how did it all start? How did you end up working with rotifers and aquaculture?
AH: In Japan, undergraduates usually enter a research lab in their fourth year and conduct research for a year as part of their graduation requirement. During this time, I did a sediment survey in Lake Hamana, near the university’s experimental station. I discovered golden, shining resting eggs of rotifers among the sediments, which strongly inspired me. This discovery sparked my interest in the dormancy and hatching mechanisms of these resting eggs, marking the beginning of my research on rotifers. Later, during my graduate studies, mass culture and preservation of rotifers became cutting-edge topics in aquaculture, so I focused on resting eggs as a preservation method and based my doctoral dissertation on uncovering the mechanisms behind resting egg formation, dormancy, and hatching.
As a postdoctoral researcher, I spent two years at the Oceanic Institute in Hawaii, working on a project on spawning and rearing milkfish and grey mullet larvae. They were struggling to achieve stable mass cultures of both rotifers and phytoplankton, so I was hired as a specialist. This project marked my entry into aquaculture.
HFM: Rotifers were first used as live feed in marine fish hatcheries in the 1960s. How did this happen?
AH: Before the 1960s, larvae rearing from fertilized eggs to juvenile fish was achieved using wild-caught zooplankton or artificially hatched bivalve or barnacle larvae in Japan. However, the difficulty in securing large quantities of live feed limited the number of juveniles that could be produced. Rotifers were initially seen as nuisance organisms that affected water quality in eel farming ponds, and research focused on removing them. However, a researcher studying the ecology of sweetfish (ayu) discovered that its larvae grew by feeding on rotifers, opening the door for rotifers as feed organisms. This research was conducted by Professor Takashi Ito of Mie Prefectural University.
HFM: How has rotifer culture evolved since then? What have been the main advancements and challenges?
AH: Initially, cultivating rotifers required developing technology to mass-produce phytoplankton, which needed large areas—5 to 10 times the size of rotifer tanks. Research to create cost-effective methods for large-scale microalgae cultivation was essential. Using agricultural fertilizers like ammonium sulfate and superphosphate enabled low-cost cultivation, allowing the method to spread nationwide. Small private hatcheries, however, still faced challenges since they lacked the space for cultivating microalgae for rotifer feeding.
Baker’s yeast was introduced as a rotifer food in the late 1960s. It was discovered that commercially available baker’s yeast could support high-density cultures of over 100 rotifers per mL, enabling some facilities to produce over 100,000 fry in a single season. Dr. Hachiro Hirata was instrumental in introducing agricultural fertilizers and baker’s yeast.
The next challenge was that yeast-based rotifer cultures often led to unstable growth and frequent culture failures, affecting fry rearing stability. Dr. Takeshi Watanabe, studying fish nutrition, identified that the lack of ω3-HUFA, including EPA and DHA, was the cause. Enriching the rotifers with these nutrients before feeding them to the fry led to significant improvements in fry growth, survival, and vitality. This spurred the development of nutritional enrichment products for hatcheries.

Atsushi Hagiwara graduate student days at the University of Tokyo. On the left is Prof. Charles E. King from Oregon State University, a rotifer specialist in evolutionary biology, and on the right is Prof. Akinori Hino, Hagiwara supervisor. Credits: Atsushi Hagiwara
HFM: What are the main rotifer culture techniques currently available for hatchery farmers?
AH: The primary methods include batch, semi-continuous, and continuous culture, with continuous culture being the most stable and semi-continuous the least stable but most cost-effective. Hatcheries choose the type based on tank size, rotifer type, and budget. In Japan, high-density mass culture became widespread in the 1990s, particularly for S-type and SS-type rotifers, where rotifers are cultured at densities of several thousand per mL or more. Concentrated freshwater Chlorella products, rich in vitamin B12 and even DHA and EPA, have been a game-changer in achieving these high densities and stable cultures.
HFM: Is instability still an issue in rotifer mass culture? Is handling still the key to stable production?
AH: The causes of instability in rotifer culture include changes in the bacterial community, increases in un-ionized ammonia, and contamination by harmful protozoa. Among these factors, the most significant impacts are attributed to changes in the bacterial community and the proliferation of harmful bacteria. However, even though monitoring the culture environment is essential, real-time bacterial community monitoring technology is not yet available.
Continuous culture is the most effective method to maintain a stable environment, and using probiotics that suppress harmful bacteria has been a viable approach. The ideal would be a continuous culture in a sterile environment, though this is currently challenging to implement at hatcheries.
As a cost-effective method for stable rotifer culture, extensive continuous culture should be cited. This method was introduced in Japan about 25 years ago and has become widespread in hatcheries equipped with large tanks (over 10 tons in size), particularly suitable for the production of L-type rotifers.
HFM: Are there differences in rotifer production systems across species, countries, or markets?
AH: In terms of rotifer species, high-density cultures at several thousand individuals per milliliter can be applied to S-type rotifers, but achieving such densities with L-type rotifers is challenging. Hatchery farmers should select the appropriate size of rotifer according to the mouth size of the fish larvae. Additionally, the optimal habitat temperatures for different fish species vary, as do the ambient temperatures of the production facilities, which can limit the types of rotifers that can be cultured.
In Southeast Asia and Africa, there are examples of extensive culture methods in outdoor tanks, where fertilization is done to cultivate feed algae and rotifers. While the production of rotifers is limited to small tanks, this method is cost-effective.
In my lab, we’ve developed rotifer culture methods using fish waste as a feed source, in collaboration with students from Southeast Asia and Africa. By adding specific probiotics, we have achieved stable cultures. This approach is low-cost but also nutritionally advantageous since fish waste is rich in omega-3 HUFA, making them nutritionally optimal for fish feed.
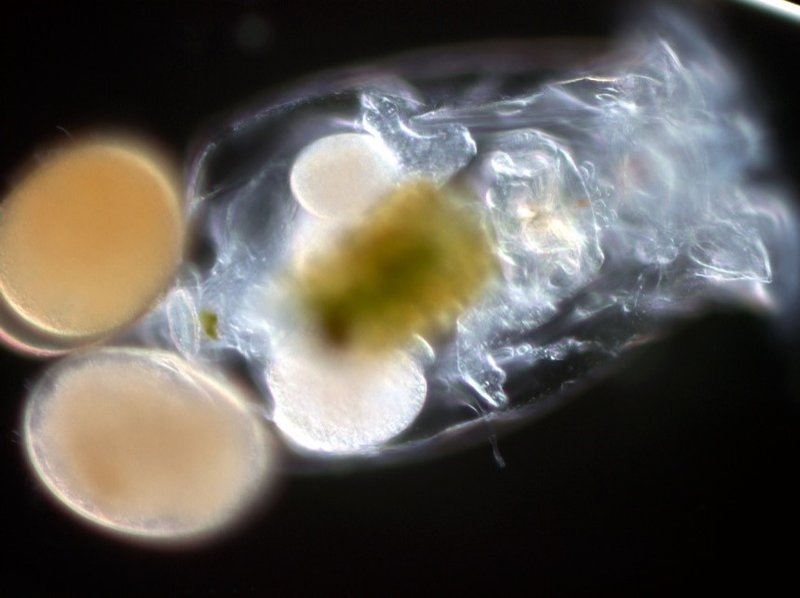
Credits: Atsushi Hagiwara
HFM: What impact would automation, monitoring tools, and AI have on rotifer culture?
AH: If aquaculture technicians understand rotifer biology and cultivation techniques, AI-driven automation can help reduce labor. However, we haven’t yet reached a point where rotifer cultivation can be fully automated. Monitoring tools can support technicians, but it’s crucial to remain hands-on with cultivation and not rely solely on technology, as this can hinder the ability to detect and prevent culture issues. It is crucial to leverage high-tech equipment only after thoroughly understanding rotifer cultivation.
Rotifer behavior parameters such as rotifer swimming speed, the frequency of directional changes, feeding behavior, and attachment behavior are related to rotifer physiological condition and stability of rotifer cultures. The development of automated technologies that measure them could potentially detect the collapse of rotifer cultures in advance. Furthermore, if we could routinely monitor gene expression associated with culture performance, it would enable even earlier detection of culture collapse.
One additional point is the color of rotifer culture water and the condition of bubbles forming on the water surface which may also be related to the stability of rotifer cultures. Skilled technicians use these indicators to assess the quality of rotifer cultures. Currently, these indicators are based on empirical observations, and there is a lack of scientific evidence supporting them. The size and number of bubbles forming on the water surface are associated with the viscosity of the culture water, but the effects of water viscosity on rotifer reproduction and swimming have only been partially reported by me around 30 years ago.
I developed a technique in the 1990s to rapidly measure the enzyme activity of rotifers to accurately predict culture instability a few days in advance. However, the associated costs and labor were prohibitive, preventing its application in practice.
HFM: What are the main challenges and opportunities for fish/shrimp hatchery production in the next few years?
AH: From a scientific perspective, numerous critical issues have been raised but remain unresolved. Among these, egg quality stands out as one of the most pivotal, as it directly impacts not only the hatching success of fertilized eggs but also the overall quality of larvae. Egg quality is heavily influenced by both the nutritional status and genetic traits of the broodstock, yet our understanding of the genetic component remains limited. By advancing our knowledge of egg quality through molecular genetics, we could make significant strides in enhancing productivity within the aquaculture industry.
For aquaculture companies, establishing breeding lines with superior traits is a fundamental challenge. Fortunately, the rapid advancements in molecular genetics have paved the way for genomic breeding technologies, allowing for more efficient and precise selective breeding. Efforts to develop lines with traits such as high growth rates and disease resistance are already underway. By integrating traits that improve egg quality, we could unlock substantial productivity gains for the aquaculture sector.